Viral Vector Development Service Market Analysis & Forecast 2025-2031
Viral Vector Development Service Market by Product Type ( Retroviral Vectors, Lentiviral Vectors, Adenoviral Vectors, Adeno-associated Viral Vectors, vaccinia vector, herpes simplex victor, baculovirus vector) by Application / End User (Pharmaceutical Manufacturers, Biotechnology Companies, Research Institutes) by Industry Analysis, Volume, Share, Growth, Challenges, Trends, and Forecast 2025-2031, Regional Outlook ( North America, Europe, Asia-Pacific, Middle-East, Africa)
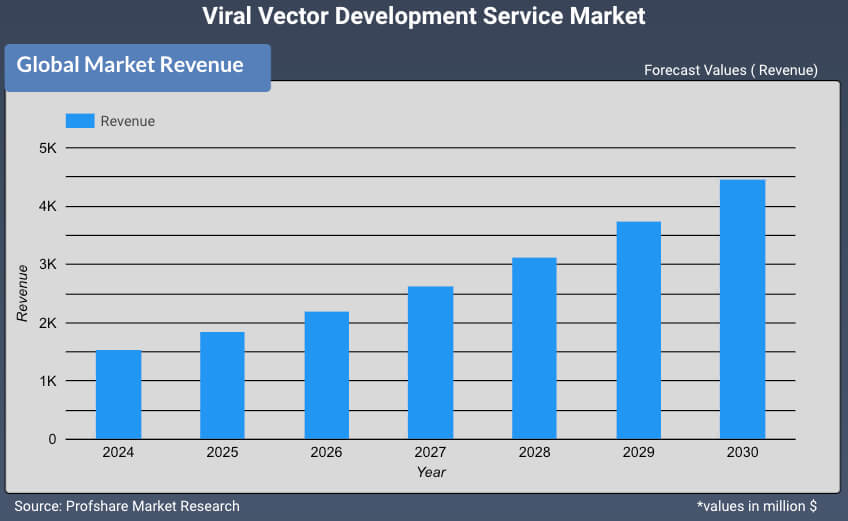
Global Viral Vector Development Service Market is expected to reach USD 4483.35 million by 2031 with CAGR of 19.2 % between 2025 and 2031
Viral vector is gene transfer therapy use to manipulate and modify specific cell type or tissue. It is most effective gene manipulation technique used globally. Various virus types are use in Viral vector process to provide either transient or permanent gene delivery to cells. Viral vector process based on virus included are:
- Retroviral Vectors - Retroviruses are stably integrate their genomes into host cell chromosomes. These viruses are positive strand RNA viruses and can enter virtually any mammalian cell type. Retroviruses can carry foreign genes of around 8 kb.
- Lentiviral Vectors - Lentiviruses are a subgroup of the retrovirus family and allow stable, long-term expression into the host cell genome.
- Adenoviral Vectors - Adenoviruses are DNA viruses can transiently transduce nearly any mammalian cell type. The adenovirus enters target cells by binding to the Coxsackie/Adenovirus receptor (CAR) . The packaging capacity of adenoviruses is 7–8 kb.
- Adeno-associated Viral Vectors - Adeno-associated viruses are capable of transducing a broad range of dividing and non-dividing cells types, with a helper virus like adenovirus or herpes virus to produce recombinant virions in packaging cells. Adeno-associated viruses have packaging capacity of up to 4.9 kb.
- Other Viral Vectors - Other viral vector systems based on vaccinia virus, herpes simplex virus, baculovirus.
Viral Vectors technique relies completely on viruses . The choice of virus for Viral Vectors process depend on :
- Safety: Pathogenic viruses are used occasionally to create viral vectors, they are modified in specific way to minimize handling risk.
- Low toxicity: The viral vector should have as minimal as possible effect on tissue or cell it infects. It has vital importance in studies requiring gene delivery in vivo as immune response will get developed in organism against foreign cell body.
- Stability: Some viruses are genetically unstable and can rapidly rearrange their genomes. Hence unstable vectors are usually avoided in Viral Vectors process.
- Cell type specificity: Most viral vectors are constructed to infect as wide a range of cell types as possible and sometimes the opposite is preferred.
- Selection: Viral vectors should contain selectable properties like resistance to a certain antibiotic, so that the cells that have taken up the viral vector can be isolated.
Viral Vector Development Service Market : End User/Application
- Pharmaceutical Manufacturers
- Biotechnology Companies
- Research Institutes
Viral Vector Development Service Market : Product Type
- Retroviral Vectors
- Lentiviral Vectors
- Adenoviral Vectors
- Adeno-associated Viral Vectors
- Other Viral Vectors
Viral Vector Development Service Market : Competition Analysis
This reports covers in-depth analysis of major players of Viral Vector Development Service Market. Competition Analysis included in study covers Company Profile, Products, Services and Solutions, Viral Vector Development Service Revenue (Value), Recent Developments . Some of key players in market are:- Kaneka Eurogentec
- FinVector
- Brammer Bio
- Cell and Gene Therapy Catapult
- FUJIFILM Diosynth Biotechnologies
- Sanofi
- Spark Therapeutics
- Cobra Biologics
- UniQure and MassBiologics
- Renova Therapeutics
- Shenzhen SiBiono GeneTech
- Thermo Fisher Scientific
Global Viral Vector Development Service Market: Regional Analysis
-
North America
- U.S.A
- Canada
- Europe
- France
- Germany
- Spain
- UK
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South East Asia
- Latin America
- Brazil
- Middle East and Africa
Global Viral Vector Development Service Market Report delivers a comprehensive analysis of the following parameters:
- Market Forecast for 2025-2031
- Market growth drivers
- Challenges and Opportunities
- Emerging and Current market trends
- Market player Capacity, Production, Revenue (Value)
- Supply (Production), Consumption, Export, Import analysis
- End user/application Analysis
Report Coverage
| Parameters | Details |
|---|---|
Base Year |
2025 |
Historical Data |
2019-2024 |
Forecast Data |
2025-2031 |
Base Year Value (2024) |
USD 1311.2 million |
Forecast Value (2031) |
USD 4483.35 million |
CAGR (2025-2031) |
19.2 % |
Regional Scope |
North America, Europe, Asian Pacific, Latin America, Middle East and Africa, and ROW |
Frequently Asked Questions (FAQ)
Viral Vector Development Service Market was valued at around USD 1311.2 million in 2024 & is estimated to reach USD 4483.35 million by 2031.
Viral Vector Development Service Market is likely to grow at Compound Annual Growth Rate (CAGR) of 19.2% between 2025-2031.
Viral Vector Development Service Market is dominated by the Retroviral Vectors segment and the North America region holds the highest market share in 2023
Some of the top key players of the Viral Vector Development Service Market are Kaneka Eurogentec,FinVector,Brammer Bio,Cell and Gene Therapy Catapult,FUJIFILM Diosynth Biotechnologies,Sanofi,Spark Therapeutics,Cobra Biologics,UniQure and MassBiologics,Renova Therapeutics,Shenzhen SiBiono GeneTech,Thermo Fisher Scientific
Primary driving factors for the growth of the Viral Vector Development Service Market include Rising cases of the cancer patients along with technological development in the healthcare industry
Yes, the report includes Geopolitical impact on the market.
Trending Biotechnology Reports
Cell Harvesting System Market Report Competitive Analysis, Revenue, Growth Strategies, Latest Trends, Regional Outlook ( North America, Europe, Asia-Pacific, Middle-East, Africa) and Forecast 2025-2031
Infection Surveillance Systems Market Report Competitive Analysis, Revenue, Growth Strategies, Latest Trends, Regional Outlook, Geopolitical Impact and Forecast 2025-2031
High Mobility Group Protein B1 Market Report Competitive Analysis, Revenue, Growth Strategies, Latest Trends, Regional Outlook ( North America, Europe, Asia-Pacific, Middle-East, Africa) and Forecast 2025-2031
Competent Cells Market Report Competitive Analysis, Revenue, Growth Strategies, Latest Trends, Regional Outlook ( North America, Europe, Asia-Pacific, Middle-East, Africa) and Forecast 2025-2031
We are committed to offering 100 % free customization while purchasing.